Innovative vaccine delivery technology

Industry partner
Vaxxas Pty Ltd

Research organisation
University of Sydney

Manufacturing Investment
$3,910,917
($556,230 IMCRC)
for 2018-2022
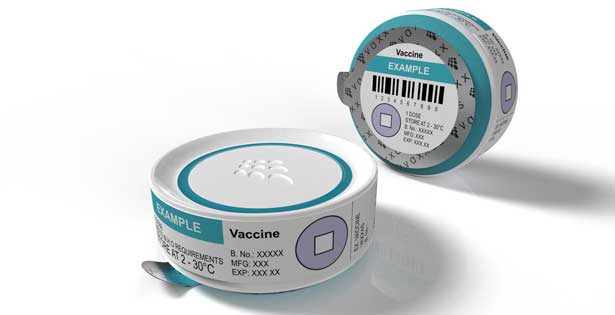
The needle-free future of vaccination
Challenge
Commercialisation of a new medical application is a lengthy and expensive process. Before investing into the next development phase of its vaccine delivery technology, Vaxxas wants to verify the intended product design through end-user testing and assessment of supply chain logistics.
Proposed Solution
In collaboration with the University of Sydney, Vaxxas aims to de-risk its manufacturing investments by undertaking studies to test a prototype of the intended commercial Vaxxas vaccine applicator.
Over the course of two years researchers will carry out
- A supply chain logistics impact / disruption assessment to highlight the cost-effectiveness of the vaccine delivery technology, the environmental sustainability and potential Industry 4.0 applications.
- An end-user usability study to ensure that the vaccine delivery technology meets clinician and patient requirements
- An acceptability study as part of a Phase 1 clinical study to gather information how well this novel, needle-free vaccination technology is received by subjects.
The results of these studies will enable Vaxxas to initiate Australian based pilot scale manufacturing knowing that the product design satisfies end user and logistical requirements for its intended markets.
